Clinical Project Manager New Protocol Template
Clinical Project Manager New Protocol Template - However, others may also find this template. Amended clinical trial protocol version no. Investigators for such trials are strongly encouraged to use this template when developing protocols for nih supported clinical trial(s). Nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types of research: Welcome to global health trials' tools and templates library. The protocol templates contain instructions and example text that can be included in your protocol. After reading, you will understand how to find a relevant clinical. Background improving the quality of patient care remains a global necessity. Share team news and announcements; Add your own steps, milestones, and dates for a comprehensive, expansive view. Site capabilities highlight frequently used resources; None of the templates are likely to be perfect for a given. The protocol templates contain instructions and example text that can be included in your protocol. After reading, you will understand how to find a relevant clinical. Background improving the quality of patient care remains a global necessity. Send due date reminders using the work progress tracker template with automation features Investigators for such trials are strongly encouraged to use this template when developing protocols for nih supported clinical trial(s). This template helps you embrace flexibility and promote collaboration with all stakeholders. The mop is developed to facilitate consistency in protocol implementation and data. Add your own steps, milestones, and dates for a comprehensive, expansive view. In this blog, you have access to the links to the clinical trial protocol template from several regulatory bodies. After reading, you will understand how to find a relevant clinical. Nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types of research: Share team news and announcements; A project activity. In this blog, you have access to the links to the clinical trial protocol template from several regulatory bodies. Please note that this page has been updated for 2015 following a quality check and review of the templates, and many new ones. Despite system and professional benefits, current evidence indicates that the spread of. Site capabilities highlight frequently used resources;. Site capabilities highlight frequently used resources; After reading, you will understand how to find a relevant clinical. Phase 2 or 3 clinical trials that require. Use this template to develop your own clinical trial timeline. It's therefore important for a project manager to develop a realistic. Nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types of research: Phase 2 or 3 clinical trials that require. Please note that this page has been updated for 2015 following a quality check and review of the templates, and many new ones. Clinical trial protocol template this protocol template. Step 2 of the ich harmonized guideline on the clinical electronic structured harmonized protocol (cesharp) occurred in september 2022. Share team news and announcements; Please note that this page has been updated for 2015 following a quality check and review of the templates, and many new ones. Table 2 of the third schedule (page. A project activity list is a. This template helps you embrace flexibility and promote collaboration with all stakeholders. This page includes seven different protocol templates for developing a variety of different new research protocols. Investigators for such trials are strongly encouraged to use this template when developing protocols for nih supported clinical trial(s). The protocol templates contain instructions and example text that can be included in. This page includes seven different protocol templates for developing a variety of different new research protocols. Please note that this page has been updated for 2015 following a quality check and review of the templates, and many new ones. None of the templates are likely to be perfect for a given. In this blog, you have access to the links. Use this template to develop your own clinical trial timeline. Amended clinical trial protocol version no. It's therefore important for a project manager to develop a realistic. Clinical trial protocol template this protocol template is designed to help research teams develop a clinical trial protocol that includes an investigational intervention (drug, biologic,. In this blog, you have access to the. Clinical trial protocol template this protocol template is designed to help research teams develop a clinical trial protocol that includes an investigational intervention (drug, biologic,. Please note that this page has been updated for 2015 following a quality check and review of the templates, and many new ones. Add your own steps, milestones, and dates for a comprehensive, expansive view.. Share team news and announcements; In this blog, you have access to the links to the clinical trial protocol template from several regulatory bodies. None of the templates are likely to be perfect for a given. Phase 2 or 3 clinical trials that require. Send due date reminders using the work progress tracker template with automation features It's therefore important for a project manager to develop a realistic. Nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types of research: Use this template to develop your own clinical trial timeline. After reading, you will understand how to find a relevant clinical. Amended clinical trial protocol version no. This page includes seven different protocol templates for developing a variety of different new research protocols. The protocol templates contain instructions and example text that can be included in your protocol. In this blog, you have access to the links to the clinical trial protocol template from several regulatory bodies. Despite system and professional benefits, current evidence indicates that the spread of. However, others may also find this template. The mop is developed to facilitate consistency in protocol implementation and data. Clinical trial protocol template this protocol template is designed to help research teams develop a clinical trial protocol that includes an investigational intervention (drug, biologic,. Provided below are standard templates that can be used by researchers to develop and design their study protocol: Background improving the quality of patient care remains a global necessity. Share team news and announcements; Please note that this page has been updated for 2015 following a quality check and review of the templates, and many new ones.Clinical Trial Project Management Plan Template
Clinical Trial Project Management Plan Template
Minimal Risk Protocol Template INSTRUCTIONS
Clinical Project Manager Cover Letter Template in Google Docs, Word
Clinical Protocol Template Master of Documents
Free Clinical Trial Templates Smartsheet
Clinical Study Protocol Template
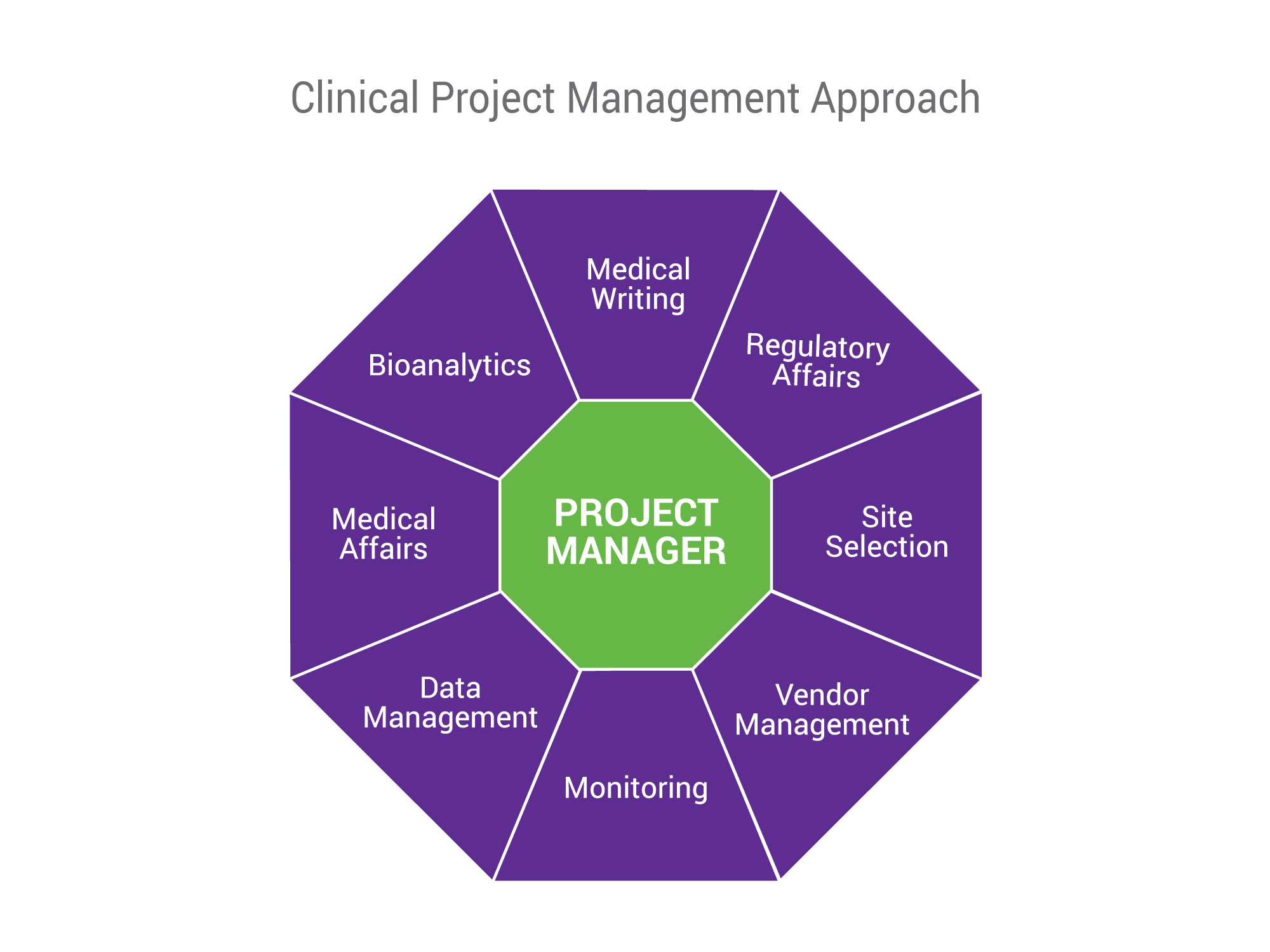
Clinical Project Management Nutrasource
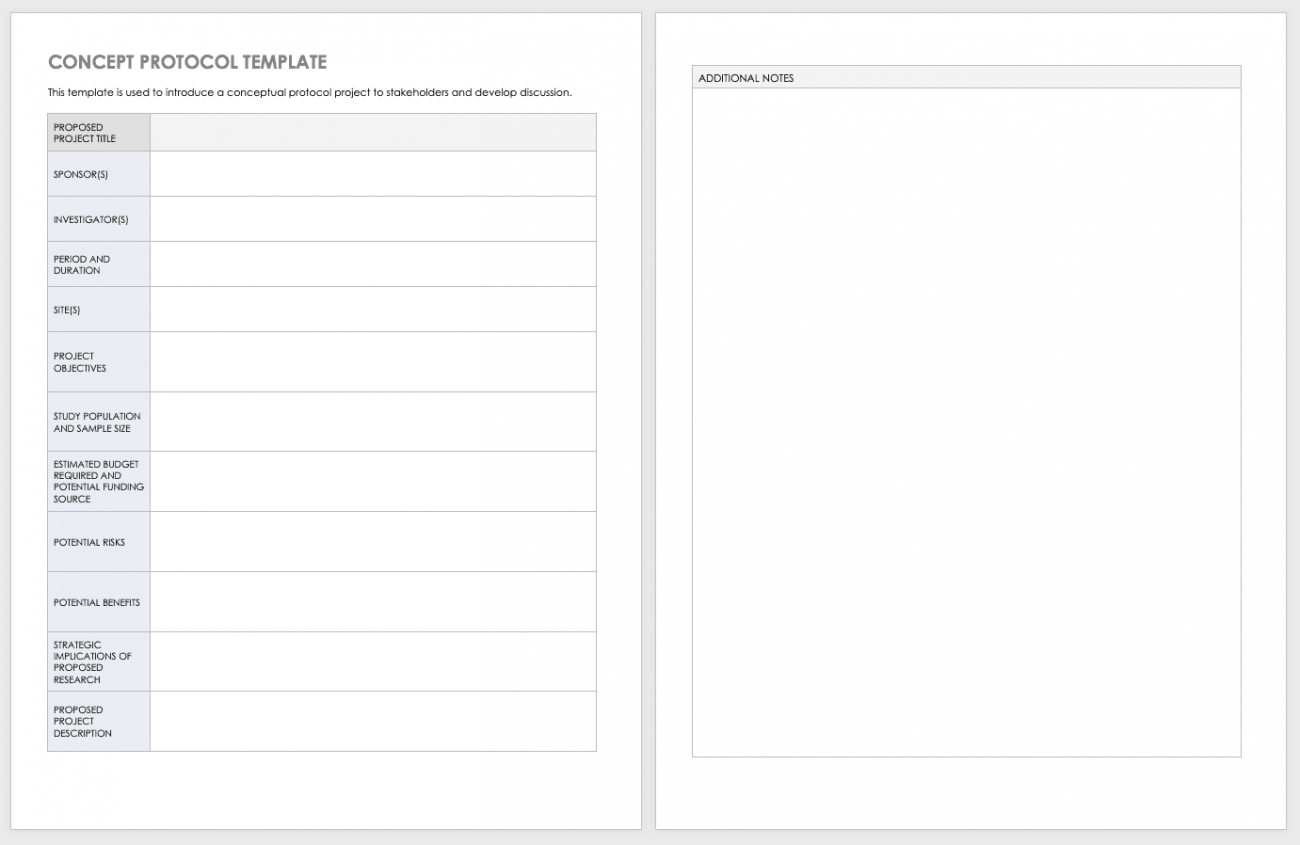
Sample Research Protocol PDF Clinical Trial Risk
Free Clinical Trial Templates Smartsheet
Table 2 Of The Third Schedule (Page.
This Template Helps You Embrace Flexibility And Promote Collaboration With All Stakeholders.
Site Capabilities Highlight Frequently Used Resources;
A Project Activity List Is A Detailed, Itemized.
Related Post: