Clinical Trial Protocol Template
Clinical Trial Protocol Template - Congenital heart disease (chd) is. It ensures consistency across clinical trial sites and adherence to regulatory and ethical. This clinical trial protocol template is a suggested format for phase 2 or 3 clinical trials supported by the national institutes of health (nih) that are being conducted under a food. The study protocol has been registered in the clinicaltrials.gov protocol registration system, identification number nct05698277. Includes protocol structure, organization, and guidance for nidcd u01 applications and fda. This report presents the explanation and elaboration paper for the consort (consolidated standards of reporting trials) 2010 and spirit (standard protocol items:. Fgs provided statistical expertise in clinical trial design. Cfaam, bgp, fgs, dsmp, and pmr conceived the idea and study design. The protocol is the backbone of your clinical trial, detailing every step of the study. Clinical trial protocol cena713din01 / nct02989402 a prospective, 16 week, phase iv study to evaluate safety, tolerability and effectiveness in patients with severe dementia of Please note that this page has been updated for 2015 following a quality check and review of the templates, and many new ones. Clinical trials conducted after regulatory submission of a dossier, but prior to the medicine's approval and launch. You can download a clinical trial protocol template in word from the national dutch ethics committee, the ccmo, website. Background prostate cancer (pca) is the most common male malignancy in the western world. Cfaam, bgp, fgs, dsmp, and pmr conceived the idea and study design. Clinical trials are intended in their broadest sense and means any study design that involves an intervention regardless of the nature of that intervention (drug, biologic, device. A protocol template for a phase iv clinical trial of secukinumab 300 mg and 150 mg in adult patients with active psoriatic arthritis. Clinical trial protocol and protocol amendment(s) (see 6.), the ich guideline for structure and content of clinical study reports, and other appropriate ich. The protocol is the backbone of your clinical trial, detailing every step of the study. The electronic protocol writing tool aims to facilitate the development of two types of clinical trials involving human participants. A protocol template for a phase iv clinical trial of secukinumab 300 mg and 150 mg in adult patients with active psoriatic arthritis. The following protocol templates are available to assist you in developing a standalone protocol: For biomedical clinical investigations evaluating drugs and/or devices, the following templates. This clinical trial protocol template is a suggested format for phase 2. The protocol is the backbone of your clinical trial, detailing every step of the study. Clinical trials are intended in their broadest sense and means any study design that involves an intervention regardless of the nature of that intervention (drug, biologic, device. Welcome to global health trials' tools and templates library. Research study protocol template (for clinical trials) instructions this. Clinical trial protocol and protocol amendment(s) (see 6.), the ich guideline for structure and content of clinical study reports, and other appropriate ich. For biomedical clinical investigations evaluating drugs and/or devices, the following templates. Congenital heart disease (chd) is. Phase 2 or 3 clinical trials that require. Includes protocol structure, organization, and guidance for nidcd u01 applications and fda. Clinical trials are intended in their broadest sense and means any study design that involves an intervention regardless of the nature of that intervention (drug, biologic, device. Cfaam wrote the draft version. Trials is experimenting with a new way of structuring study protocols for randomised trials. The protocol includes study objectives, design,. Includes protocol structure, organization, and guidance for nidcd. The study protocol has been registered in the clinicaltrials.gov protocol registration system, identification number nct05698277. Includes protocol structure, organization, and guidance for nidcd u01 applications and fda. The following protocol templates are available to assist you in developing a standalone protocol: For biomedical clinical investigations evaluating drugs and/or devices, the following templates. These trials may supplement earlier trials, complete earlier. Congenital heart disease (chd) is. Background prostate cancer (pca) is the most common male malignancy in the western world. There are two templates to be used for interventional research: For biomedical clinical investigations evaluating drugs and/or devices, the following templates. This clinical trial protocol template is a suggested format for phase 2 or 3 clinical trials supported by the national. Includes protocol structure, organization, and guidance for nidcd u01 applications and fda. Cfaam wrote the draft version. Clinical trial protocol and protocol amendment(s) (see 6.), the ich guideline for structure and content of clinical study reports, and other appropriate ich. Clinical trial protocol cena713din01 / nct02989402 a prospective, 16 week, phase iv study to evaluate safety, tolerability and effectiveness in. There are two templates to be used for interventional research: This clinical trial protocol template is a suggested format for phase 2 or 3 clinical trials supported by the national institutes of health (nih) that are being conducted under a food. The study protocol has been registered in the clinicaltrials.gov protocol registration system, identification number nct05698277. For biomedical clinical investigations. Clinical trial protocol cena713din01 / nct02989402 a prospective, 16 week, phase iv study to evaluate safety, tolerability and effectiveness in patients with severe dementia of Welcome to global health trials' tools and templates library. This clinical trial protocol template is a suggested format for phase 2 or 3 clinical trials supported by the national institutes of health (nih) that are. This report presents the explanation and elaboration paper for the consort (consolidated standards of reporting trials) 2010 and spirit (standard protocol items:. The study protocol has been registered in the clinicaltrials.gov protocol registration system, identification number nct05698277. You can download a clinical trial protocol template in word from the national dutch ethics committee, the ccmo, website. The protocol includes study. You can also find a template on the websites of. These trials may supplement earlier trials, complete earlier trials, or may. This clinical trial protocol template is a suggested format for phase 2 or 3 clinical trials supported by the national institutes of health (nih) that are being conducted under a food. The electronic protocol writing tool aims to facilitate the development of two types of clinical trials involving human participants. It ensures consistency across clinical trial sites and adherence to regulatory and ethical. There are two templates to be used for interventional research: Welcome to global health trials' tools and templates library. Please note that this page has been updated for 2015 following a quality check and review of the templates, and many new ones. The interventional drug/device trial template and the behavioral and social science research template both. Nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types of research: The simple innovation is to include all 51 spirit headings and item identifiers within the protocol. The study protocol has been registered in the clinicaltrials.gov protocol registration system, identification number nct05698277. For biomedical clinical investigations evaluating drugs and/or devices, the following templates. This report presents the explanation and elaboration paper for the consort (consolidated standards of reporting trials) 2010 and spirit (standard protocol items:. Clinical trials conducted after regulatory submission of a dossier, but prior to the medicine's approval and launch. Phase 2 or 3 clinical trials that require.Ich Format for a Clinical Trial Protocol Clinical Trial Statistics
Sample Research Protocol PDF Clinical Trial Risk
Clinical Trial Protocol Template Word
Phase 1 Clinical Trial Protocol Template
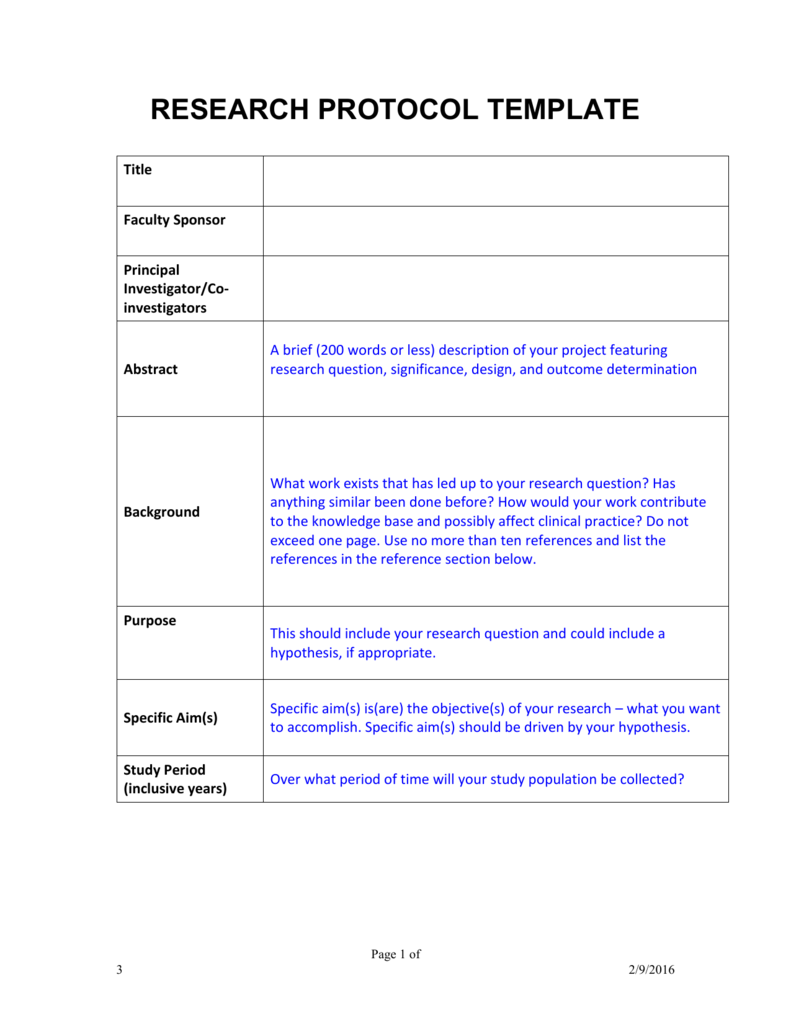
research protocol template
Free Clinical Trial Templates Smartsheet
Free Clinical Trial Templates Smartsheet
Clinical Study Protocol (CSP) Template Clinical Study Templates
Clinical Study Protocol Template
Phase 1 Clinical Trial Protocol Template
Clinical Trials Are Intended In Their Broadest Sense And Means Any Study Design That Involves An Intervention Regardless Of The Nature Of That Intervention (Drug, Biologic, Device.
The Protocol Includes Study Objectives, Design,.
Background Prostate Cancer (Pca) Is The Most Common Male Malignancy In The Western World.
Cfaam, Bgp, Fgs, Dsmp, And Pmr Conceived The Idea And Study Design.
Related Post: