Consort Diagram Template
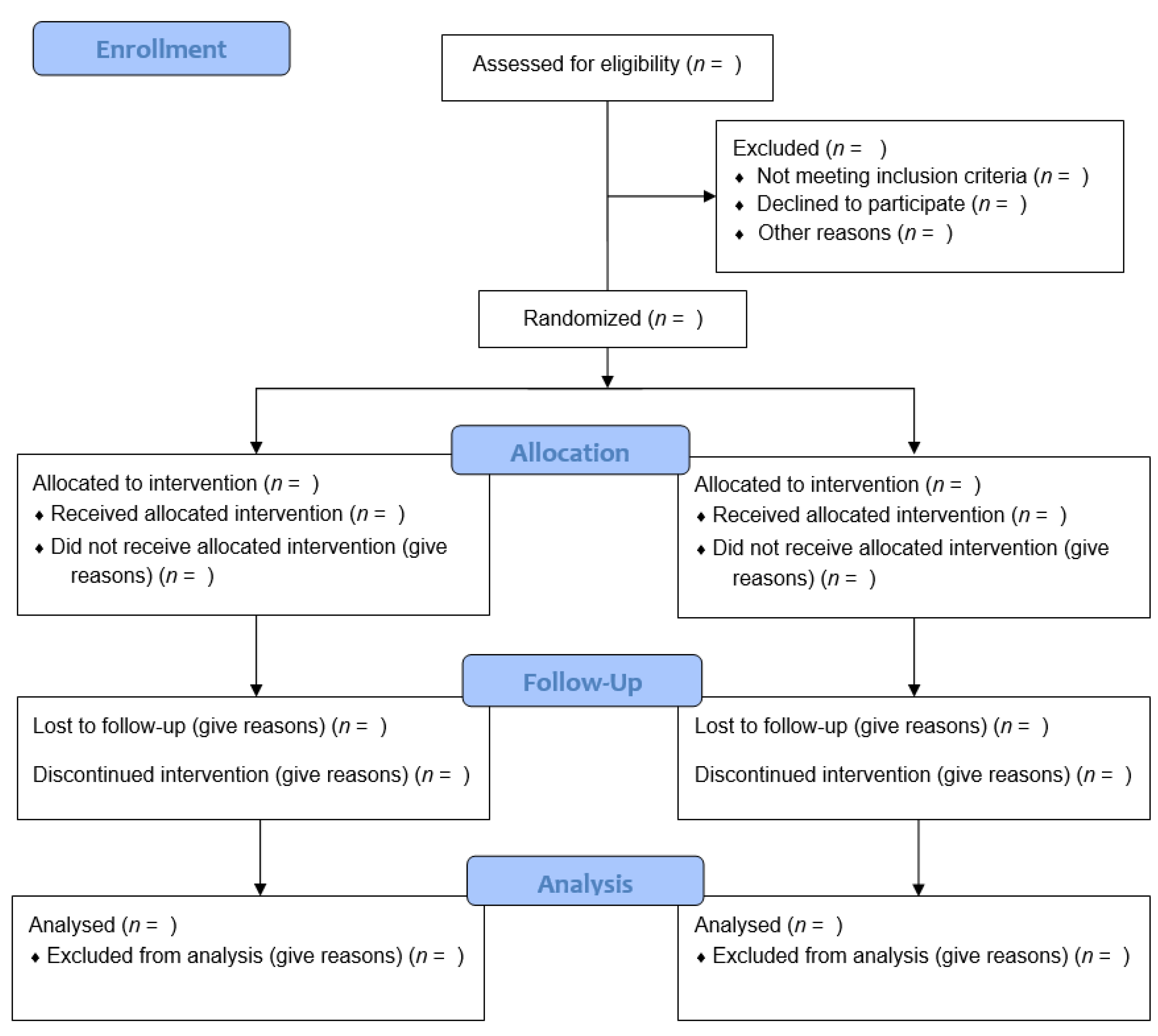
Consort Diagram Template - Analysed (n= ) excluded from analysis (give reasons) (n= ) allocated to intervention (n= ). Not meeting inclusion criteria (n= ) ♦. Use creately’s easy online diagram editor to edit this diagram, collaborate with others and export results to multiple image formats. Sample template for the consort diagram showing the flow of participants through each stage of a randomized trial last modified by: Hpb consort diagram template assessed for eligibility n = randomized n = excluded n = did not meet inclusion criteria n = refused to participate n = other reasons n = allocated to. 50.9 kb ) for free. Assessed for eligibility (n= ) excluded (n= ) ♦. Other reasons (n= ) analysed (n= ) ♦. This file contains the consort 2010 flow diagram and checklist used for reporting randomized trials. Consort 2010 explanation and elaboration: This file contains the consort 2010 flow diagram and checklist used for reporting randomized trials. Consort 2010 explanation and elaboration: Assessed for eligibility (n= ) excluded (n= ) ♦. You can easily edit this template using creately. It provides essential information on trial design, methods, results, and conclusions. Sample template for the consort diagram showing the flow of participants through each stage of a randomized trial author: Sample template for the consort diagram showing the flow of participants through each stage of a randomized trial last modified by: Updated guidelines for reporting parallel group randomised trial. Analysed (n= ) excluded from analysis (give reasons) (n= ) allocated to intervention (n= ). Download or preview 1 pages of pdf version of sample template for the consort diagram (doc: Use creately’s easy online diagram editor to edit this diagram, collaborate with others and export results to multiple image formats. Not meeting inclusion criteria (n= ) ♦. Hpb consort diagram template assessed for eligibility n = randomized n = excluded n = did not meet inclusion criteria n = refused to participate n = other reasons n = allocated to.. Consort 2010 explanation and elaboration: Assessed for eligibility (n= ) excluded (n= ) ♦. Analysed (n= ) excluded from analysis (give reasons) (n= ) allocated to intervention (n= ). You can easily edit this template using creately. Not meeting inclusion criteria (n= ) ♦. Sample template for the consort diagram showing the flow of participants through each stage of a randomized trial author: Declined to participate (n= ) ♦. Other reasons (n= ) analysed (n= ) ♦. You can easily edit this template using creately. The explanation and elaboration paper for this guideline was published. Assessed for eligibility (n= ) excluded (n= ) ♦. You can easily edit this template using creately. Use creately’s easy online diagram editor to edit this diagram, collaborate with others and export results to multiple image formats. It provides essential information on trial design, methods, results, and conclusions. Hpb consort diagram template assessed for eligibility n = randomized n =. This file contains the consort 2010 flow diagram and checklist used for reporting randomized trials. Declined to participate (n= ) ♦. Not meeting inclusion criteria (n= ) ♦. Updated guidelines for reporting parallel group randomised trial. Analysed (n= ) excluded from analysis (give reasons) (n= ) allocated to intervention (n= ). 50.9 kb ) for free. This file contains the consort 2010 flow diagram and checklist used for reporting randomized trials. Hpb consort diagram template assessed for eligibility n = randomized n = excluded n = did not meet inclusion criteria n = refused to participate n = other reasons n = allocated to. You can easily edit this template using. Sample template for the consort diagram showing the flow of participants through each stage of a randomized trial last modified by: The explanation and elaboration paper for this guideline was published. Hpb consort diagram template assessed for eligibility n = randomized n = excluded n = did not meet inclusion criteria n = refused to participate n = other reasons. Other reasons (n= ) analysed (n= ) ♦. Analysed (n= ) excluded from analysis (give reasons) (n= ) allocated to intervention (n= ). The explanation and elaboration paper for this guideline was published. It provides essential information on trial design, methods, results, and conclusions. Use creately’s easy online diagram editor to edit this diagram, collaborate with others and export results. Use creately’s easy online diagram editor to edit this diagram, collaborate with others and export results to multiple image formats. Assessed for eligibility (n= ) excluded (n= ) ♦. Other reasons (n= ) analysed (n= ) ♦. This file contains the consort 2010 flow diagram and checklist used for reporting randomized trials. You can easily edit this template using creately. Declined to participate (n= ) ♦. Updated guidelines for reporting parallel group randomised trial. Other reasons (n= ) analysed (n= ) ♦. Not meeting inclusion criteria (n= ) ♦. Download or preview 1 pages of pdf version of sample template for the consort diagram (doc: 50.9 kb ) for free. You can easily edit this template using creately. Consort 2010 explanation and elaboration: Sample template for the consort diagram showing the flow of participants through each stage of a randomized trial last modified by: Use creately’s easy online diagram editor to edit this diagram, collaborate with others and export results to multiple image formats. Not meeting inclusion criteria (n= ) ♦. Download or preview 1 pages of pdf version of sample template for the consort diagram (doc: This file contains the consort 2010 flow diagram and checklist used for reporting randomized trials. Sample template for the consort diagram showing the flow of participants through each stage of a randomized trial author: The explanation and elaboration paper for this guideline was published. Assessed for eligibility (n= ) excluded (n= ) ♦. Updated guidelines for reporting parallel group randomised trial. Hpb consort diagram template assessed for eligibility n = randomized n = excluded n = did not meet inclusion criteria n = refused to participate n = other reasons n = allocated to.Consort Diagram Template
Consort Diagram Template
Consort Flow Diagram Template
Consort Flow Diagram Template
Consort Diagram Template
Consort Diagram Template
Consort Flow Diagram Template
Consort Flow Diagram Template
Consort Flow Diagram Template
Consort Flow Diagram Template Word
Other Reasons (N= ) Analysed (N= ) ♦.
Analysed (N= ) Excluded From Analysis (Give Reasons) (N= ) Allocated To Intervention (N= ).
Declined To Participate (N= ) ♦.
It Provides Essential Information On Trial Design, Methods, Results, And Conclusions.
Related Post: