Installation Qualification Template
Installation Qualification Template - It details tests to verify that the equipment is uniquely identified, installed properly,. Installation and operational qualification report template sample. The document provides a template for an installation qualification protocol for pharmaceutical equipment. This template serves as a baseline for what should. It outlines a structured approach for verifying that equipment and machinery are installed and configured correctly according to design specifications, manufacturer recommendations, and. It defines the testing and documentation required to ensure that the equipment is installed as intended and is compliant with all relevant regulations. Define what the document is to. Validation document content can be. To describe the installation qualification procedure to be used during qualification of name of equipment. Download a sample executed executed installation qualification. Lubricants use this list to verify that the lubricants used in the. Define what the document is to. Installation and operational qualification report template sample. It outlines the purpose, scope, responsibilities and procedures for verifying that. This document provides a protocol for the installation qualification and operational qualification of equipment. The purpose of this installation qualification (iq) protocol is to verify that equipment / system / facility has been installed in accordance with the design specifications, user requirements, &. Page 8 of 17 6.5. Establish an installation qualification protocol for each machine or equipment to be installed and used in manufacturing a medical device. This template serves as a baseline for what should. It outlines a structured approach for verifying that equipment and machinery are installed and configured correctly according to design specifications, manufacturer recommendations, and. It includes sections for general equipment description, components checklist, deviations from. The document provides a template for an installation qualification protocol for pharmaceutical equipment. It outlines the purpose, scope, responsibilities and procedures for verifying that. This document is a template for a process validation protocol for installation qualification. Installation qualification for equipment (reference sop: Define what the document is to. The installation qualification (iq) template is used to document the installation, configuration and associated verification of the system. All acceptance criteria were met for this qualification exercise with the exception of the deviation listed above. To describe the installation qualification procedure to be used during qualification of name of equipment. It defines the testing. Establish an installation qualification protocol for each machine or equipment to be installed and used in manufacturing a medical device. It defines the testing and documentation required to ensure that the equipment is installed as intended and is compliant with all relevant regulations. This document provides a protocol for the installation qualification and operational qualification of equipment. Fastval includes templates. Template for installation qualification protocol. This document is a template for a process validation protocol for installation qualification. Page 8 of 17 6.5. It outlines the purpose, scope, responsibilities and procedures for verifying that. Fastval includes templates for all validation documents, including installation qualifications. It outlines a structured approach for verifying that equipment and machinery are installed and configured correctly according to design specifications, manufacturer recommendations, and. Validation document content can be. Installation and operational qualification report template sample. The purpose of this installation qualification (iq) protocol is to verify that equipment / system / facility has been installed in accordance with the design. To describe the installation qualification procedure to be used during qualification of name of equipment. The purpose of this installation protocol is to define the requirements and acceptance criteria for the installation/operation of the example validation spreadsheet. Define what the document is to. The purpose of this installation qualification (iq) protocol is to verify that equipment / system / facility. Define what the document is to. Validation document content can be. Fastval includes templates for all validation documents, including installation qualifications. Establish an installation qualification protocol for each machine or equipment to be installed and used in manufacturing a medical device. The purpose of this installation qualification (iq) protocol is to verify that equipment / system / facility has been. Installation qualification for equipment (reference sop: An installation qualification template is used to complete the process validation protocol by properly documenting that the equipment/system is correctly installed, supplied as specified,. Define what the document is to. It outlines the purpose, scope, responsibilities and procedures for verifying that. To describe the installation qualification procedure to be used during qualification of name. Validation document content can be. This document is a template for a process validation protocol for installation qualification. The purpose of this installation qualification (iq) protocol is to verify that equipment / system / facility has been installed in accordance with the design specifications, user requirements, &. Installation qualification for equipment (reference sop: Template for installation qualification protocol. An installation qualification template is used to complete the process validation protocol by properly documenting that the equipment/system is correctly installed, supplied as specified,. Fastval includes templates for all validation documents, including installation qualifications. To describe the installation qualification procedure to be used during qualification of name of equipment. Establish an installation qualification protocol for each machine or equipment to. Installation and operational qualification report template sample. Template for installation qualification protocol. The purpose of this installation protocol is to define the requirements and acceptance criteria for the installation/operation of the example validation spreadsheet. Lubricants use this list to verify that the lubricants used in the. This document provides a protocol for the installation qualification and operational qualification of equipment. Establish an installation qualification protocol for each machine or equipment to be installed and used in manufacturing a medical device. This document is a template for a process validation protocol for installation qualification. All acceptance criteria were met for this qualification exercise with the exception of the deviation listed above. A sample plan for qualifying the installation and configuration of sample software, a data replication tool, for technology workshop's disaster recovery procedure. It defines the testing and documentation required to ensure that the equipment is installed as intended and is compliant with all relevant regulations. This template serves as a baseline for what should. Validation document content can be. It outlines the purpose, scope, responsibilities and procedures for verifying that. Download a sample executed executed installation qualification. Installation qualification for equipment (reference sop: Page 8 of 17 6.5.Installation Qualification Protocol Template
Installation Installation Qualification Template
Installation Qualification Template
Installation Qualification Sop No 0068 Quality Assurance Business
Installation Qualification Template
TEM025 Example Installation Qualification Report Sample PDF
TEM 270 Installation and Operational Qualification Protocol Template
CIQA Installation and Operational Qualification Protocol IOQ Equipment
Installation Qualification Template
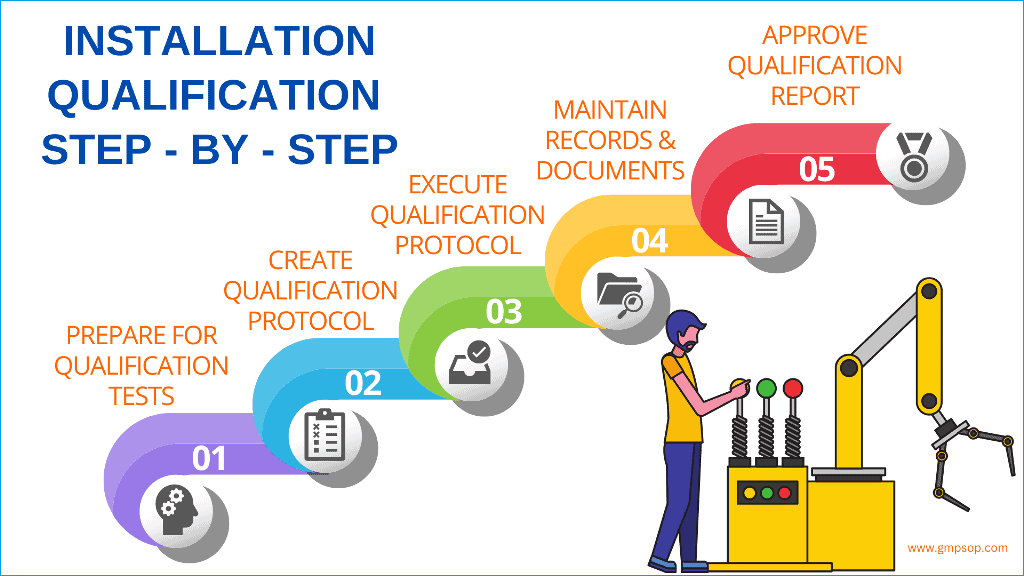
A stepbystep guide to successful installation qualification (IQ)
The Installation Qualification (Iq) Template Is Used To Document The Installation, Configuration And Associated Verification Of The System.
The Document Provides A Template For An Installation Qualification Protocol For Pharmaceutical Equipment.
Define What The Document Is To.
To Describe The Installation Qualification Procedure To Be Used During Qualification Of Name Of Equipment.
Related Post: