Irb Protocol Template
Irb Protocol Template - The irb office has developed protocol templates for use by the northwestern university research community to describe research/human research activities. Which protocol template should you use? Before preparing your application please read through the following steps to assess whether irb review is required and, if so, how to complete the eirb+ application. Getting started concerns or complaints? The irb office has developed three new protocol templates for use by the northwestern university research community to describe human research activities: It is important to use the correct template or form to ensure pertinent information is included. Biomedical & social behavioral consent templates; Write your protocol using the appropriate protocol templates from the irb’s protocol templates & forms webpage. Refer to the nu irb protocol template and address how criteria are met in the protocol Consent templates & hipaa requirements overview; The suggested consent language webpage has. Refer to the nu irb protocol template and address how criteria are met in the protocol Consent templates & hipaa requirements. The irb office has developed protocol templates for use by the northwestern university research community to describe research/human research activities. Biomedical & social behavioral consent templates; It is important to use the correct template or form to ensure pertinent information is included. Before preparing your application please read through the following steps to assess whether irb review is required and, if so, how to complete the eirb+ application. Getting started concerns or complaints? The irb office has developed three new protocol templates for use by the northwestern university research community to describe human research activities: Consent templates & hipaa requirements overview; It is important to use the correct template or form to ensure pertinent information is included. Which protocol template should you use? Biomedical & social behavioral consent templates; Consent templates & hipaa requirements overview; Write your protocol using the appropriate protocol templates from the irb’s protocol templates & forms webpage. The irb office has developed protocol templates for use by the northwestern university research community to describe research/human research activities. Consent templates & hipaa requirements. Write your protocol using the appropriate protocol templates from the irb’s protocol templates & forms webpage. Before preparing your application please read through the following steps to assess whether irb review is required and, if. Which protocol template should you use? Biomedical & social behavioral consent templates; Consent templates & hipaa requirements overview; The irb office has developed protocol templates for use by the northwestern university research community to describe research/human research activities. The suggested consent language webpage has. The suggested consent language webpage has. Getting started concerns or complaints? Worksheets are guidance materials used by irb reviewers and designated reviewers during initial reviews, continuing reviews, and modification reviews to enhance compliance with federal, state, and local requirements. Biomedical & social behavioral consent templates; Write your protocol using the appropriate protocol templates from the irb’s protocol templates & forms. Write your protocol using the appropriate protocol templates from the irb’s protocol templates & forms webpage. Consent templates & hipaa requirements. It is important to use the correct template or form to ensure pertinent information is included. Refer to the nu irb protocol template and address how criteria are met in the protocol Before preparing your application please read through. It is important to use the correct template or form to ensure pertinent information is included. Consent templates & hipaa requirements overview; Refer to the nu irb protocol template and address how criteria are met in the protocol Worksheets are guidance materials used by irb reviewers and designated reviewers during initial reviews, continuing reviews, and modification reviews to enhance compliance. Consent templates & hipaa requirements. Getting started concerns or complaints? Consent templates & hipaa requirements overview; The irb office has developed three new protocol templates for use by the northwestern university research community to describe human research activities: Write your protocol using the appropriate protocol templates from the irb’s protocol templates & forms webpage. Biomedical & social behavioral consent templates; Getting started concerns or complaints? Worksheets are guidance materials used by irb reviewers and designated reviewers during initial reviews, continuing reviews, and modification reviews to enhance compliance with federal, state, and local requirements. Refer to the nu irb protocol template and address how criteria are met in the protocol The irb office has developed. Write your protocol using the appropriate protocol templates from the irb’s protocol templates & forms webpage. Refer to the nu irb protocol template and address how criteria are met in the protocol It is important to use the correct template or form to ensure pertinent information is included. Getting started concerns or complaints? The irb office has developed three new. It is important to use the correct template or form to ensure pertinent information is included. Which protocol template should you use? Before preparing your application please read through the following steps to assess whether irb review is required and, if so, how to complete the eirb+ application. The irb office has developed protocol templates for use by the northwestern. Write your protocol using the appropriate protocol templates from the irb’s protocol templates & forms webpage. Consent templates & hipaa requirements. The suggested consent language webpage has. Before preparing your application please read through the following steps to assess whether irb review is required and, if so, how to complete the eirb+ application. It is important to use the correct template or form to ensure pertinent information is included. Biomedical & social behavioral consent templates; Refer to the nu irb protocol template and address how criteria are met in the protocol The irb office has developed three new protocol templates for use by the northwestern university research community to describe human research activities: Which protocol template should you use? The irb office has developed protocol templates for use by the northwestern university research community to describe research/human research activities.IRB Protocol and Consent ResourcesColumbia Doc Template pdfFiller
Free Irb Protocol Template Edit Online & Download
Fillable Online IRB Protocol Template Fax Email Print pdfFiller
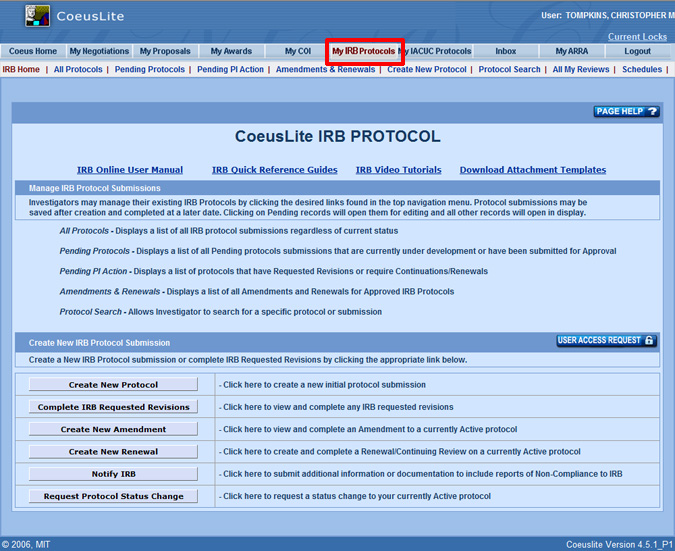
IRB Protocol Homepage Sponsored Program Services Coeus Purdue
IRB Protocol Template Center for the Enhancement of Teaching
Human Subjects IRB Protocol Doc Template pdfFiller
RP503 TEMPLATE PROTOCOL IRB Institutional Review Board
SOLUTION Template For Initial Irb Protocol Development V2 Studypool
Fillable Online IRB Working Protocol Template Clinical Trials Fax
Irb Protocol Template
Getting Started Concerns Or Complaints?
Consent Templates & Hipaa Requirements Overview;
Worksheets Are Guidance Materials Used By Irb Reviewers And Designated Reviewers During Initial Reviews, Continuing Reviews, And Modification Reviews To Enhance Compliance With Federal, State, And Local Requirements.
Related Post: